 Solved: Part A A Homeostatic Imbalance That Activates Thes... | Chegg.com
Solved: Part A A Homeostatic Imbalance That Activates Thes... | Chegg.comWarning: The NCBI website requires JavaScript to operate. Bone homeostasis Bone functions(s) are (i) mechanical support soft tissues, ii) levers for muscle action, (iii) Protection of the central nervous system, (iv) Release of calcium and other ions for maintenance of a constant ionic environment in extracellular fluid, and (v) housing and support for hemopoiesis. Structure and amount of bone, both at the macroscopic and microscopic level, determined by the genetic plane and by regulatory factors that help perform bone functions. Genetic information is responsible for the highly preserved anatomical shape of bones and probably restore that form after the fracture. To fulfill its functions, the bone suffers continuous destruction, called resorption, performed by osteoclasts, and formation by osteoblasts. In the adult skeleton, the two processes are in balance, maintaining a constant and controlled amount of bone. This in fact, as well as histological observation that the osteoclastic bone the reorption is followed by osteoblastic bone formation (), taken to the concept that the two processes are mechanically "coupled" and a quest for "coordination factors". It has not been shown that a single factor links the two processes. Existence evidence suggests that several factors are probably involved in maintenance of bone homeostasis. Growth factors found in the bone (), such like IGFs or TGFβs, were proposed to be released during resorption and start the local bone formation (, ). Factors deposited in the bone surface by osteoclasts at the end of the resorption phase was proposed start the following bone formation (). Moral factors, such as parathyroid hormone and prostaglandin E, which stimulates both bones bone reortion and formation, could increase the two processes Tandem. The action of these factors and other hormones and cytokines in It was proposed that osteoclasts should be mid-by-cell osteoblasts of lineage, that possess the cognant receptors (, ), intimately ligating osteoblast – osteoclast interaction to bone rotation. Finally, but not least, the ability of the bone to change its structure and adaptation to mechanical loads implies that mechanical forces can regulate bone reordering and formation: increased loads should increase training and reduction of the reorption while the download must have opposite effect. In fact, immobilization stimulates reortion and delete the training (for review, see ref. ), providing a clear example of "disapproval" between the two processes. The mechanism for these effects has not been completely clarified, but, again, Osteoblast, osteocyte and lining cells were proposed for mediate the mechanical signals because their location is more suitable for perceive them (). The link between bone formation and bone resorption was examined in a elegant studio of Corral et al. (), reported on this topic of the Proceedings, which uses a transgenic model demonstrate a clear separation between the two processes in 6 to 14-week mice. Using the promoter of osteocalcin, responsible for selective expression of this gene in mature osteoblasts, the authors destroyed these cells by expressing the thymidylate kinase (tk) and treat animals with gancyclovir, a toxin activated by tk. This study shows that the elimination of osteoblasts that form the bones and the detention of bone formation does not affect osteoclastic activity. The imbalance between the two processes resulted in significant bone loss, mimicking a phenotype of osteoporosis, which could be completely prevented treatment with alendronate inhibitor of osteoclasto bisphosphonate. In addition, osteoclasts generated in the culture of the bone marrow and bone cells of the calvary, obtained from the transgenic animals, resorb bone usually in vitro in presence or absence of gancyclovir, indicating that no cells are required to express osteocalcin differentiation or activity of murine osteoclasts vitro. At first glance, these findings seem to challenge current dogma and prevailing concepts on bone rotation osteoblasto/osteoclasto interaction, but yes? The findings raise two important and related issues: (i) What is the nature of bone resorption coupling to bone formation and (ii) which osteoblast cells, if any, affect Osteoclast activity? The authors were cautious in interpreting the findings and stated only that active bone and life formation The osteoblasts, at least those expressing osteocalcin, are not necessary for osteoclast activity in these mice. These conclusions are justified. completely by the data. On the broader issues raised by this study, integration of these findings in existing literature should take into account the next. At 6 years old, mice are still growing. During growth, bone form and structure are partly maintained by active reorption in the subepifiseal region (bone growth), the primary site observation and analysis in this study. Therefore, there should be a strong genetic influence on bone formation and reorption at that site during this stage, more closely related to bone structure (modification) that maintain bone mass during adult bone "remodelization", where the local link between the two processes should occur (). The second point refers to the duration of the study, which was from 4 to 8 weeks. Bone loss caused during the first 4 weeks of The osteoblast closure was enormously greater than the one that occurred during the after 4 weeks (Fig. 4 in ref. ). Lack of bone formation between the ages of 14 and 18 weeks does not seem to reduce markedly bone volume beyond (Fig. 4 in ref. ), suggesting that osteoclastic the activity has decreased considerably during that period compared to the previous four weeks. It would be of interest to measure osteoclastic surface at the end of the second 4-week gancyclovir period of treatment. If osteoclastic activity was reduced, it might suggest the influence of age (genetic?) or feedback provided by bone mass, instead of bone formation. Molecular base for changes osteoclast activity, if present, could be further investigated in this model. In the same context, complete cessation of osteoblastic bone formation doesn't seem to drive, at least within 8 weeks, to the continuous loss of the skeleton, which may be leveling to ♥50% of the initial bone volume, at the sites examined. Additional study of this phenomenon, including the longer duration of treatment and the mechanisms involved, can also be explored in this model. An unequivocal finding of this study is that expressive osteocalcin Osteoblasts are not necessary for the generation of osteoclasts and osteoclasts activity. This does not contradict a large number of previous studies, showing that osteoclast formation and activity, at least in culture, require interaction with estromal cells or osteoblast blood cells but not necessarily mature osteoblasts (). This interaction was recently proven to be mediated, at least in part, by the TNF-related molecule RANK ligand (, ).Another interesting point made in this study is the fact that, when bone rotation was practically shut down by treatment combined with gancyclovir and alendronate for 8 weeks, there was no apparent Perverse effects on skeleton. It has been assumed, mainly on theoretical terrain, which mechanical use causes fatigue damage in bone, repaired by bone remodeling, whose absence can increase the risk of fracture (). Eight weeks can be too short period to detect such effects in relatively young mice; longer studies this point would need to be evaluated. In short, this is an interesting study that applies the tools genetic engineering to a long-term search to unravel the base bone homeostasis. The novelty is that (in mice) bone formation and mature osteoblasts are not necessary for osteoclast activity, which can be influenced by cells that do not express osteocalcin or by age or amount of bone. This model could help clarify the links between bone processes formation and bone resorption, which should be present to maintain bone homeostasis. Footnotes A commentary on this article starts on page . ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA
Causes of osteoporosis What is osteoporosis? Osteoporosis is the thinning of the bones. It affects about 25 percent of women over 65 and 5 percent of men over 65, according to the . A variety of risk factors can determine their risk for the disease. Some are preventable, and some are inevitable. What causes bone thinning? The bone is living tissue with holes inside. The interior has a panal appearance. The bones affected by osteoporosis have bigger holes and are more fragile. Understanding osteoporosis begins with understanding how bones are made. You repeatedly place demands on your bones. Because of these demands, your bones are constantly remodeling. It occurs in two phases. First, special bone cells called decompose the bone. Then, other bone cells called creating a new bone. Osteoclasts and osteoblasts can coordinate well for most of their lives. Eventually, this coordination can be broken down, and osteoclasts begin to remove more bone than osteoblasts can create. When you're young, your body creates a lot of bone. In the middle of the 20s, their level is maximum. After that, you start to lose bone mass slowly as your body dissolves more bone than it is reconstructed. (PTH) is an important contributor to the bone remodeling process. High levels of PTH can activate osteoclasts and cause excessive bone decomposition. in your blood activates the release of PTH. Low levels of calcium in the blood, or , can cause high levels of PTH. It may also cause your own bone to release calcium to make sure you have enough calcium in your blood. You need: Your body will undermine your bones for calcium if you don't have enough in your blood. Getting enough calcium throughout your life is important to prevent bone thinning. In their teens and early adult years, they're building bones. Enough calcium intake at that time guarantees healthy bones later. As you age, eating enough calcium-rich foods helps reduce the amount of bone decomposition. is essential to keep calcium in your bones. Vitamin D helps you absorb calcium through your intestines. Many older adults do not receive enough vitamin D. Up to 50 percent of older adults with hip fractures have very low levels of vitamin D, according to . Without enough vitamin D, your bloodstream will not adequately take calcium in milk, calcium supplements or other sources. Low levels of vitamin D will also trigger a series of events leading to the activation of osteoclasts. It also leads to greater production of PTH, which creates even more osteoclasts. Osteoporosis is more likely to affect older women, especially white and Asian women, than men. One reason for this is the impact of the fall of estrogen levels later. A consistent level of estrogen is important to maintain the pace of bone remodeling. If estrogen levels decrease, this changes the levels of certain chemicals of messaging that help maintain a healthy balance of bone production and degradation. The osteoclasts become more active without estrogen, and their body breaks down more bone. Some medical conditions and some medicines can accelerate the process of osteoporosis. This is called . It occurs more often as a result of taking . as and directly delaying osteoblasts and accelerating osteoclasts. They make it harder for your body to absorb calcium, and also increase how much calcium you lose in the urine. Taking can also increase the risk of thinning the bones. Thyroid hormones accelerate the process of bone remodeling. This increase in speed results in a greater probability of imbalance between osteoblasts and osteoclasts. Taking alcohol, smoking and eating disorder are additional risk factors for osteoporosis. These interfere with their ability to absorb the necessary nutrients, such as calcium and vitamin D.The complex interactions between PTH, calcium and vitamin D maintain the balance of bone manufacturing and bone destruction cells. Some health and medicine conditions may affect the process of bone remodeling and lead to bone thinning. Maintaining the necessary levels of calcium and vitamin D is key to reducing the risk of developing osteoporosis. Last medical review on August 7, 2019Read this following

Chapter 6-Bones and Skeletal Tissue Diagram | Quizlet
IJMS | Free Full-Text | Does Allergy Break Bones? Osteoporosis and Its Connection to Allergy | HTML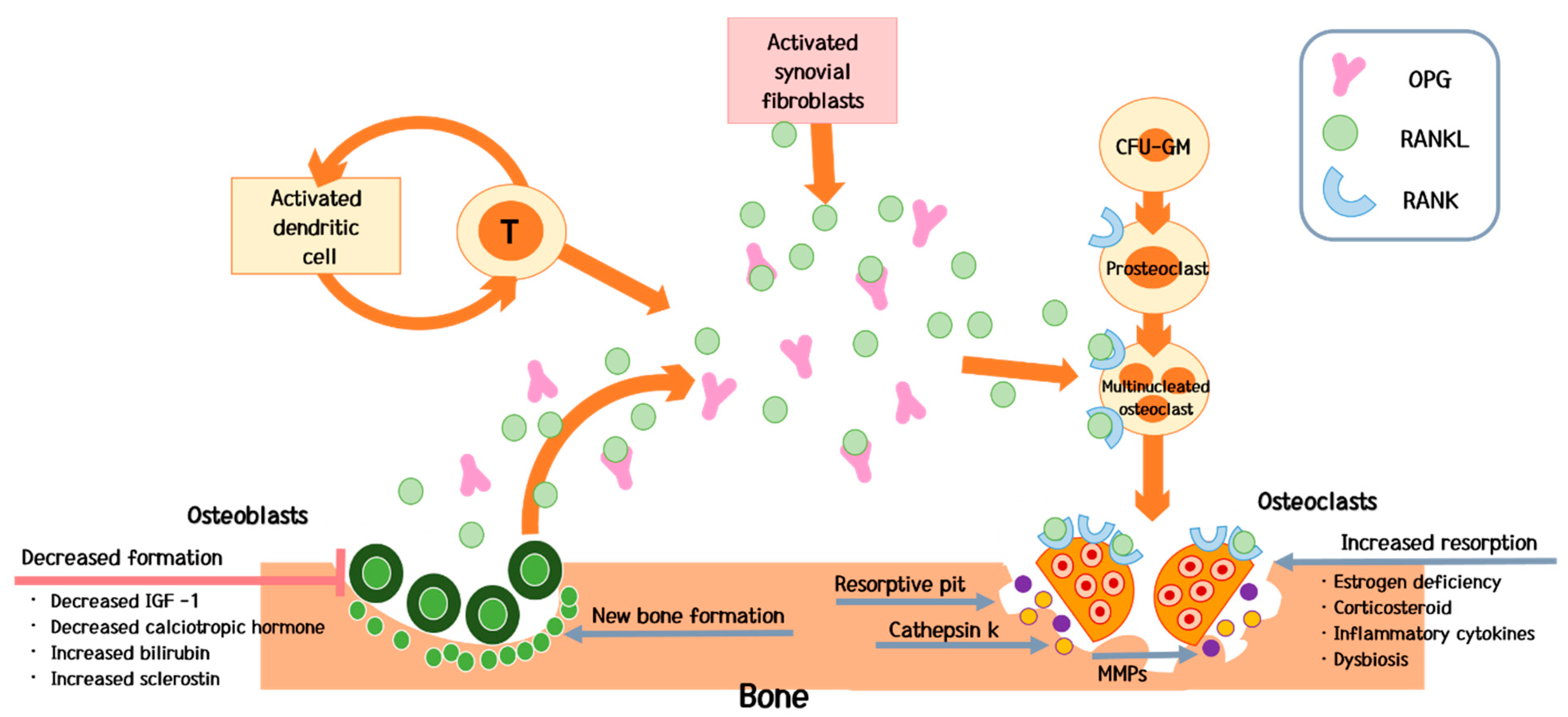
IJMS | Free Full-Text | Bone Diseases in Patients with Chronic Liver Disease | HTML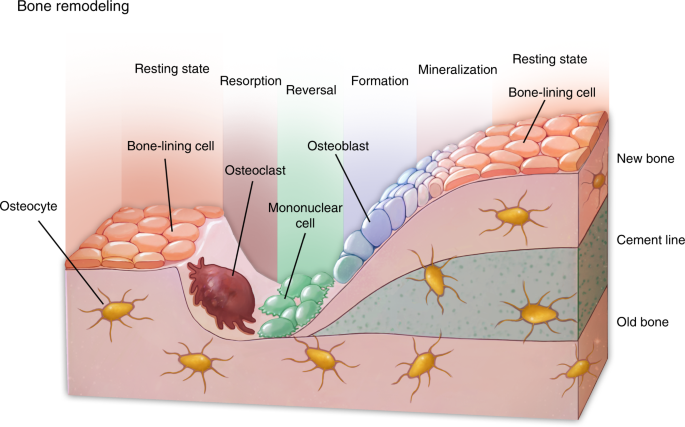
Autophagy in bone homeostasis and the onset of osteoporosis | Bone Research
Skeletal Homeostasis - an overview | ScienceDirect Topics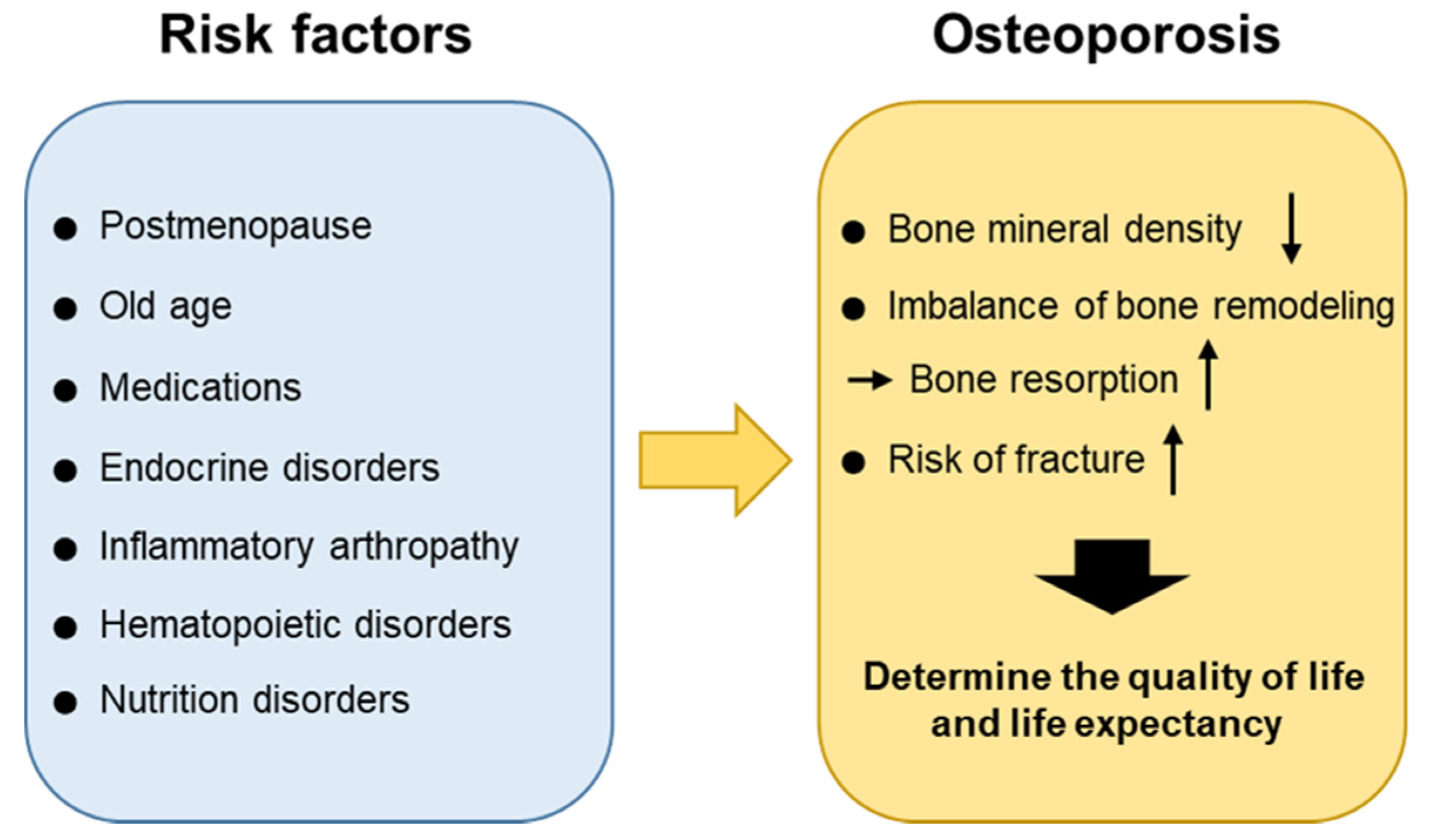
IJMS | Free Full-Text | Molecular Mechanisms and Emerging Therapeutics for Osteoporosis | HTML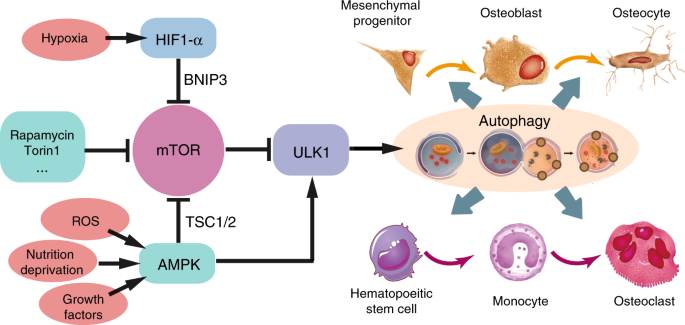
Autophagy in bone homeostasis and the onset of osteoporosis | Bone Research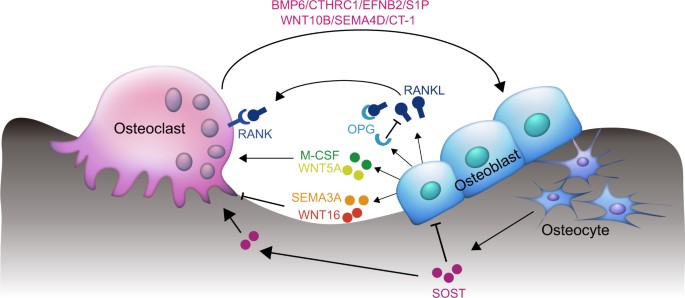
Paracrine and endocrine actions of bone—the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts | Bone Research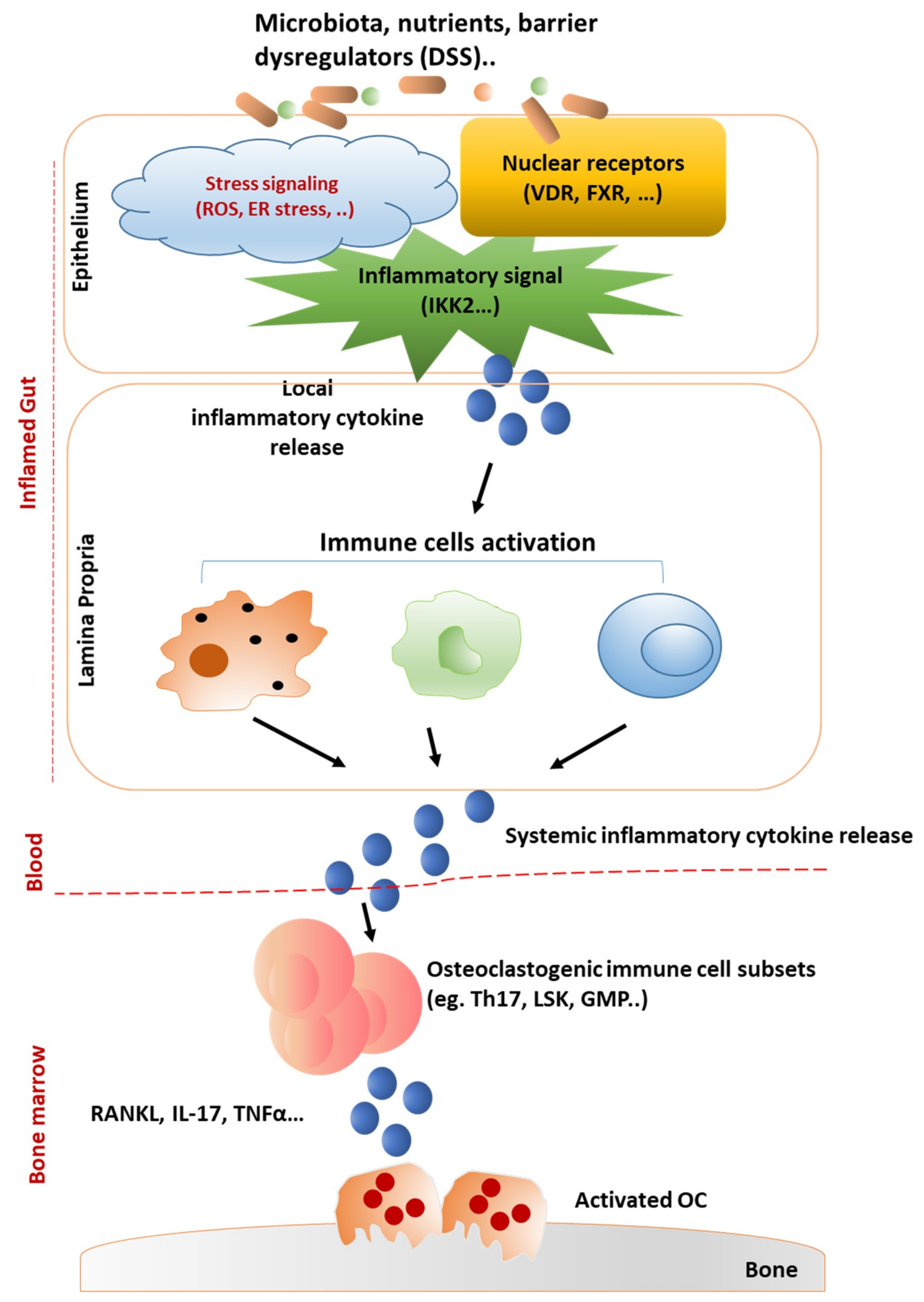
IJMS | Free Full-Text | Mechanisms Underlying Bone Loss Associated with Gut Inflammation | HTML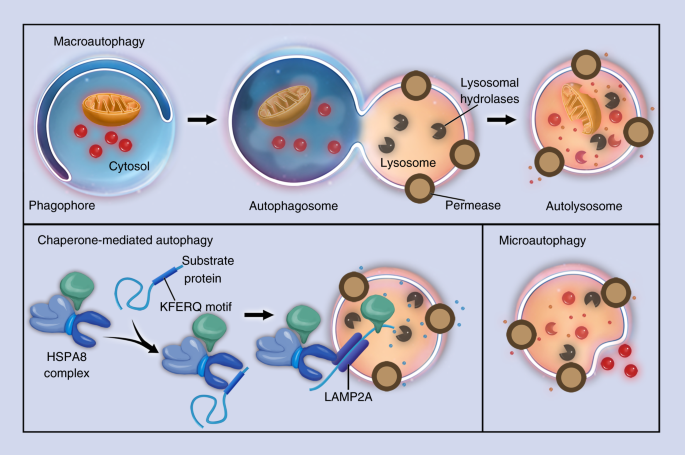
Autophagy in bone homeostasis and the onset of osteoporosis | Bone Research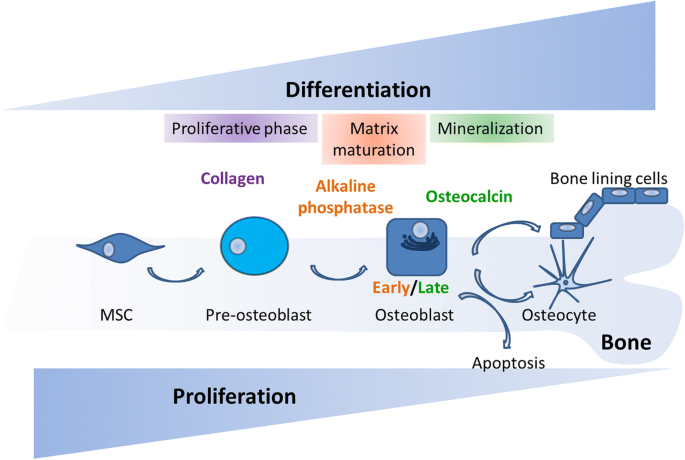
Osteogenesis and aging: lessons from mesenchymal stem cells | Stem Cell Research & Therapy | Full Text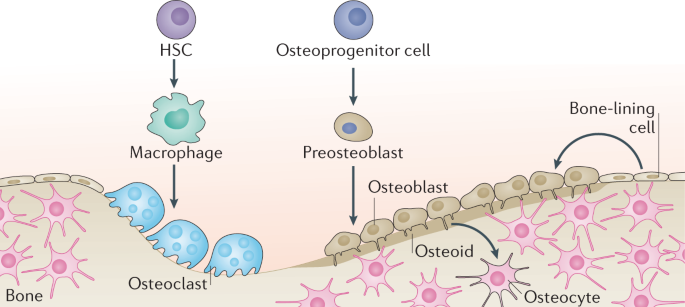
Mechanisms of bone development and repair | Nature Reviews Molecular Cell Biology
Linking bone cells, aging, and oxidative stress: Osteoblasts, osteoclasts, osteocytes, and bone marrow cells - ScienceDirect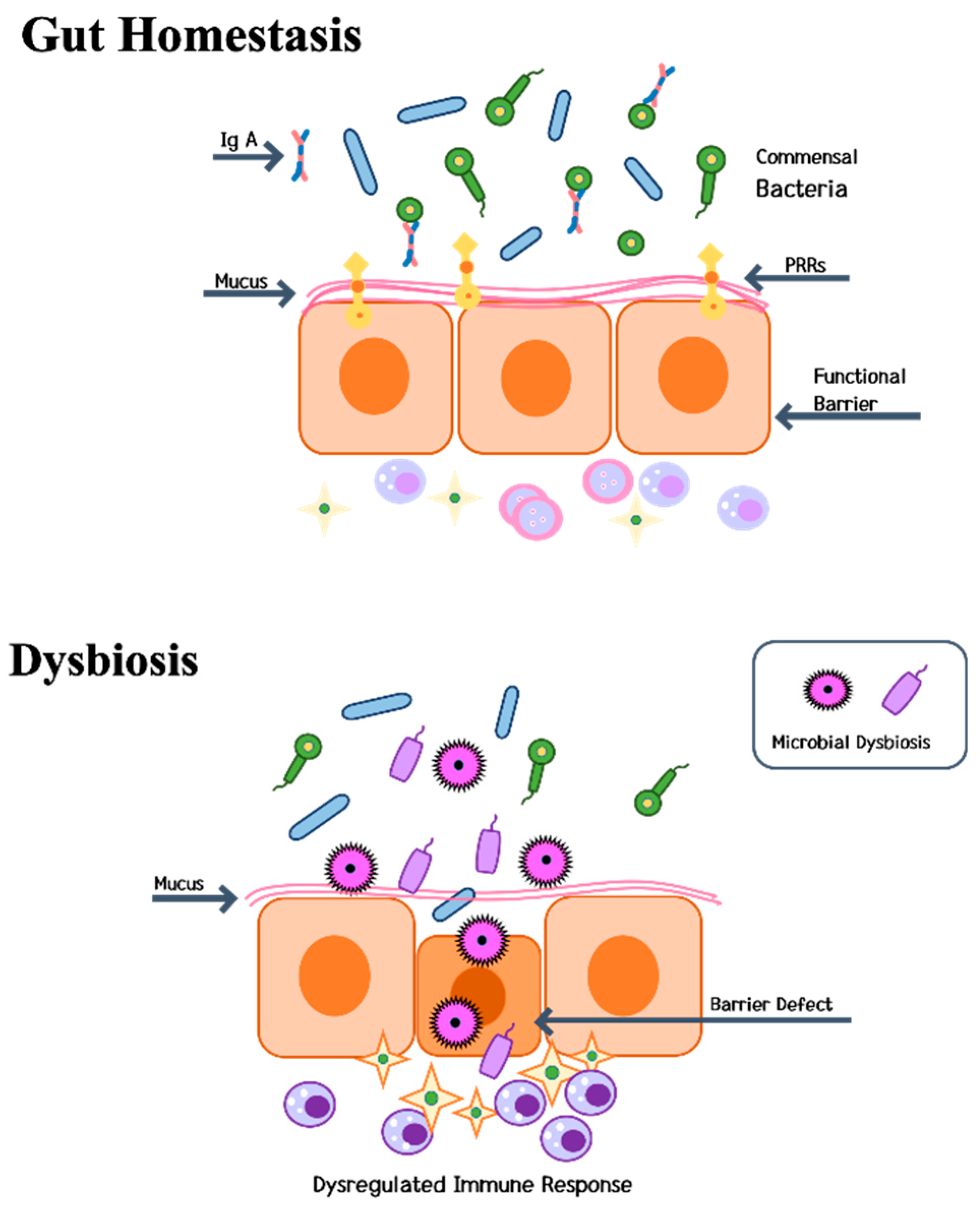
IJMS | Free Full-Text | Bone Diseases in Patients with Chronic Liver Disease | HTML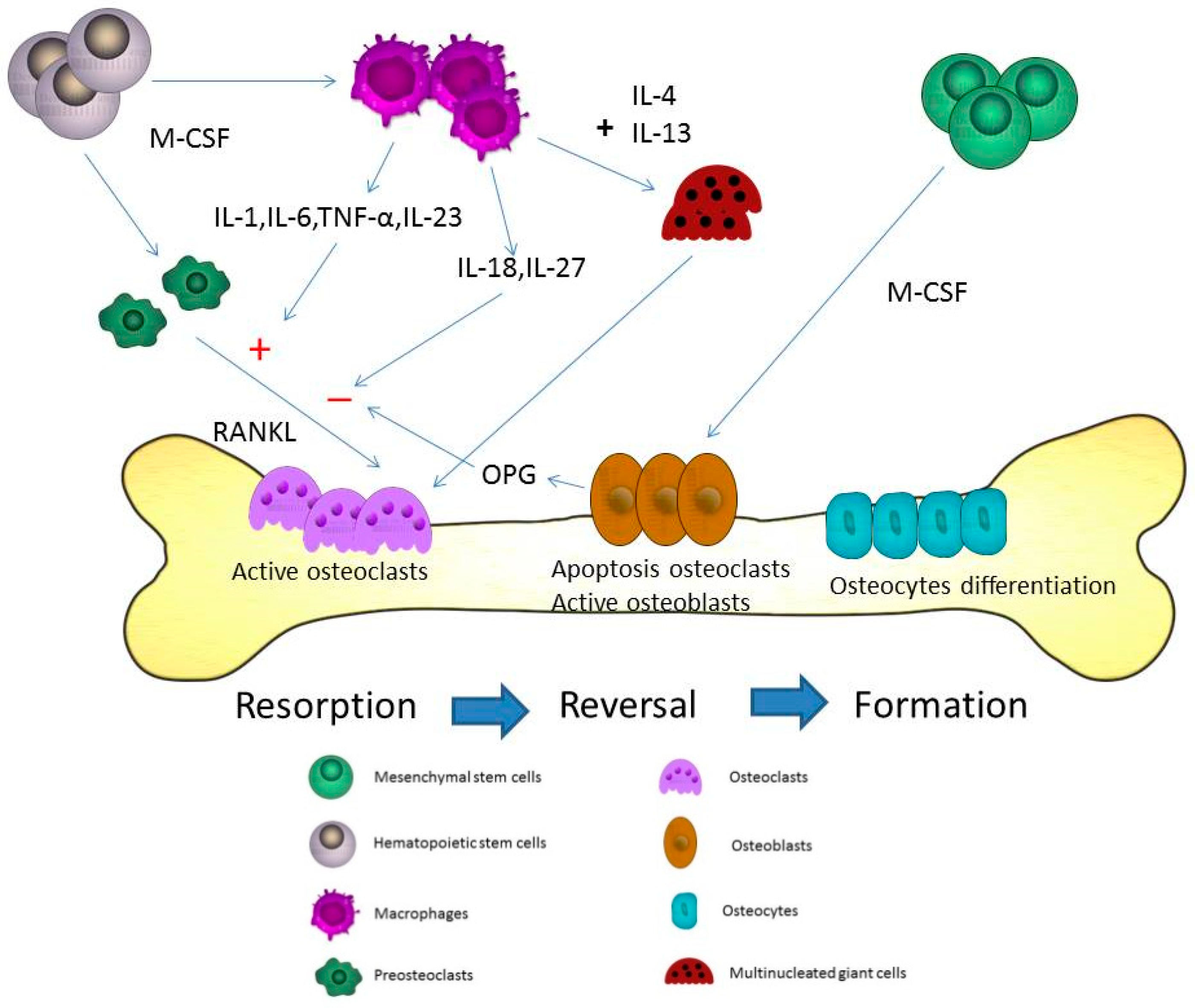
IJMS | Free Full-Text | The Role of Macrophage in the Pathogenesis of Osteoporosis | HTML
Mastering A&P: Bone Flashcards | Quizlet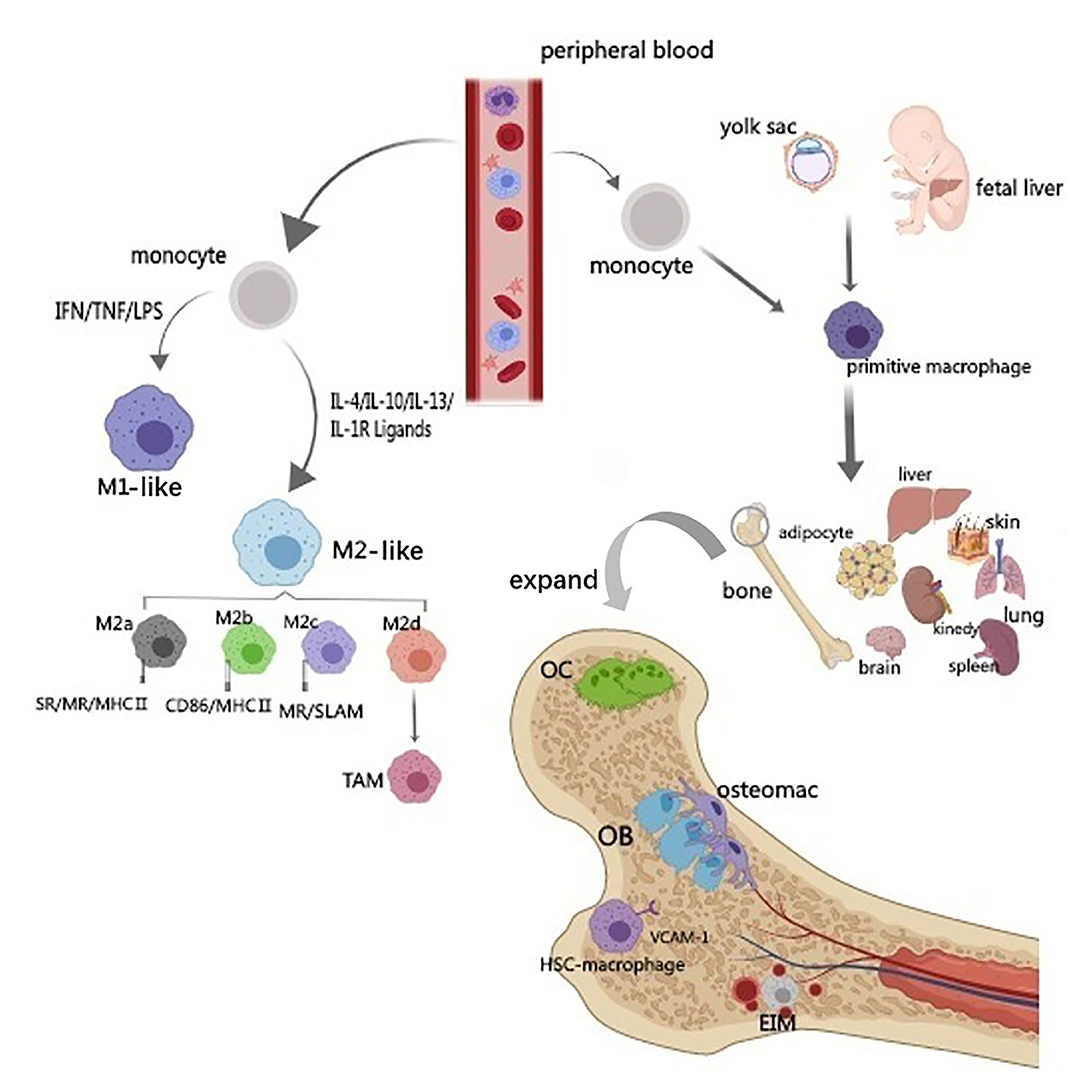
Frontiers | Communications Between Bone Marrow Macrophages and Bone Cells in Bone Remodeling | Cell and Developmental Biology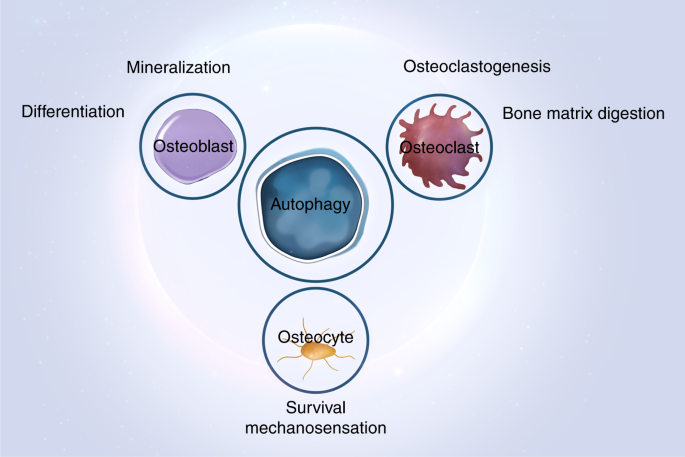
Autophagy in bone homeostasis and the onset of osteoporosis | Bone Research
P2X7 receptor acts as an efficient drug target in regulating bone metabolism system - ScienceDirect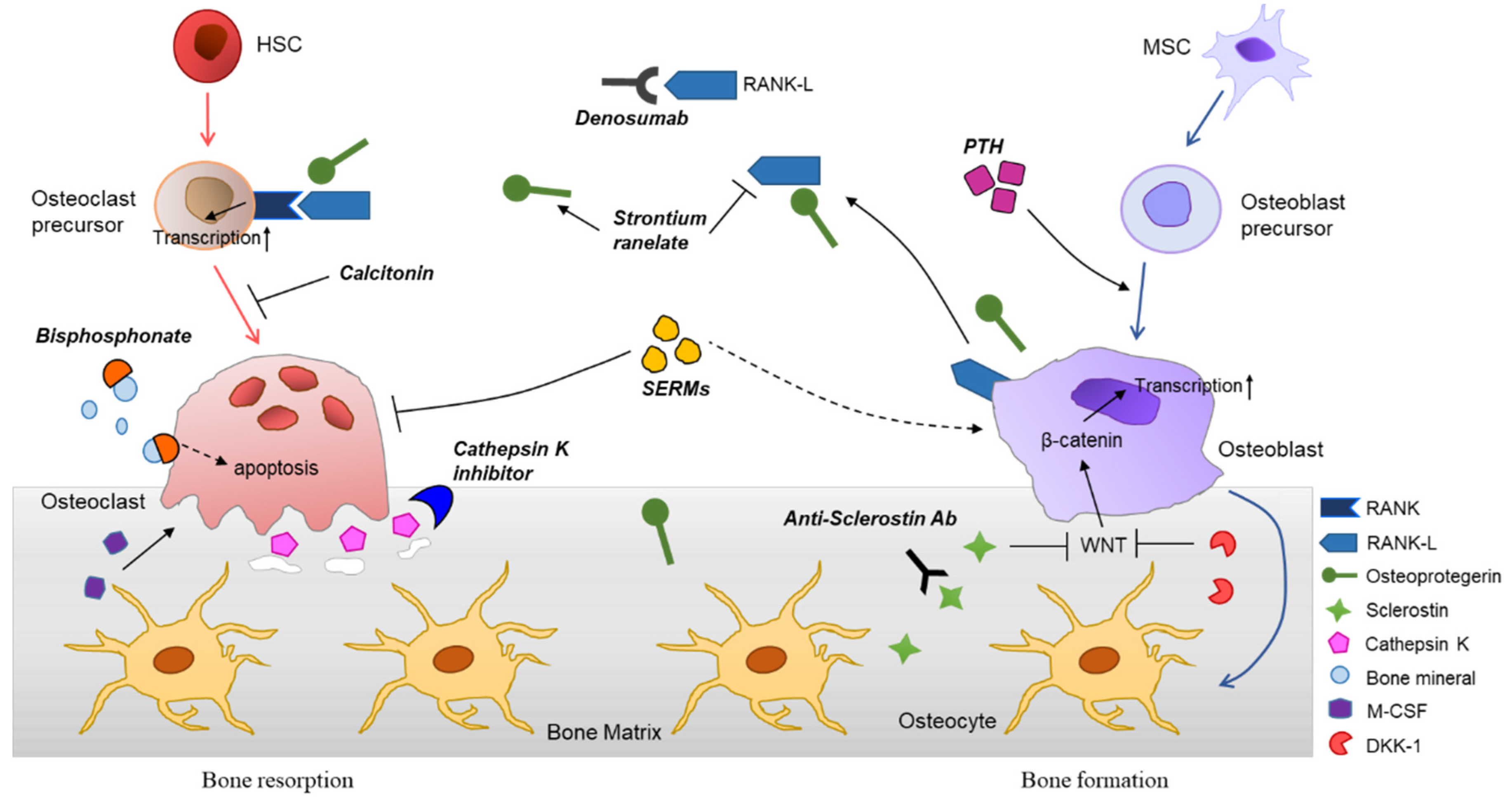
IJMS | Free Full-Text | Molecular Mechanisms and Emerging Therapeutics for Osteoporosis | HTML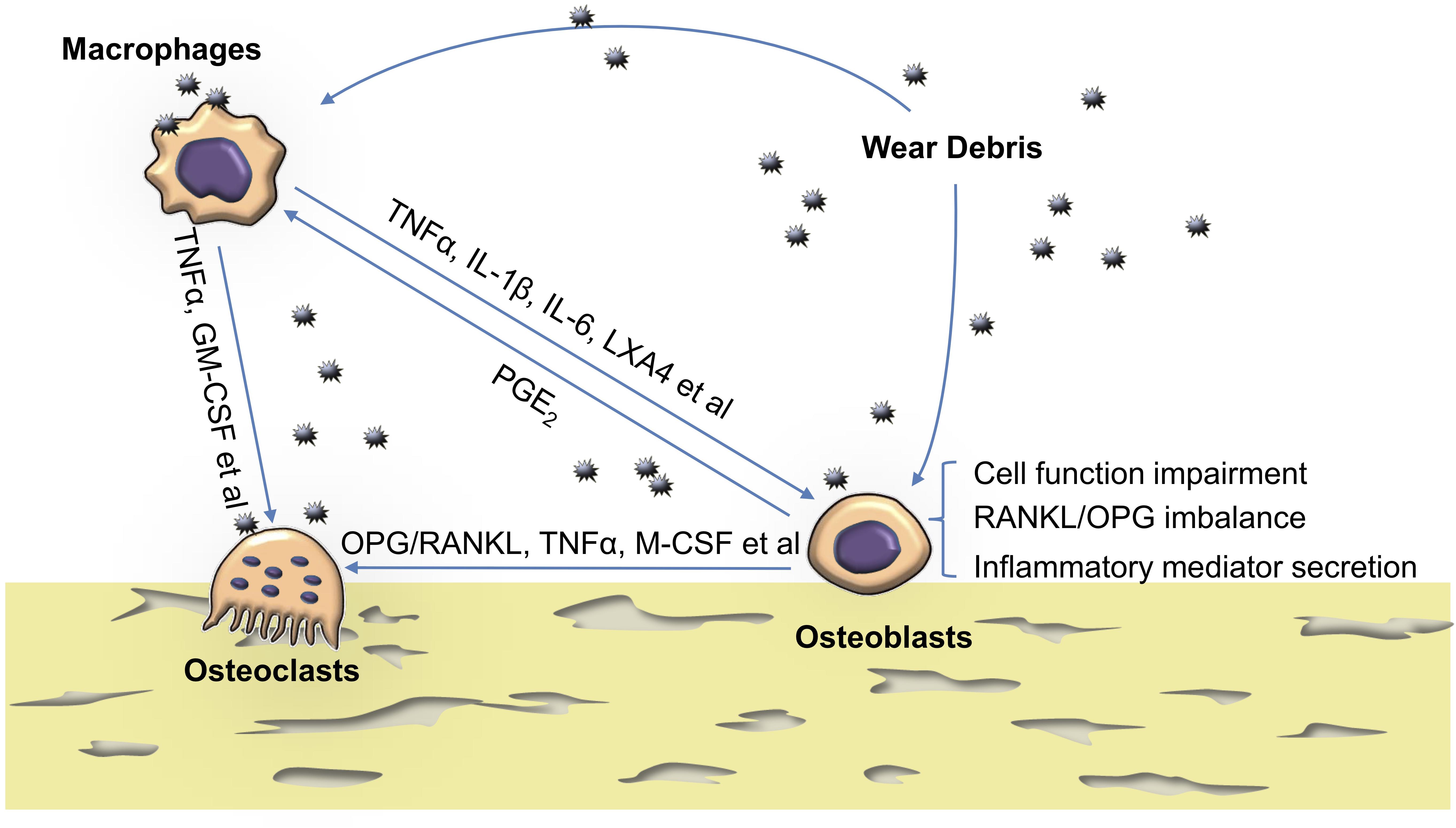
Frontiers | The Effects of Biomaterial Implant Wear Debris on Osteoblasts | Cell and Developmental Biology
In silico experiments of bone remodeling explore metabolic diseases and their drug treatment | Science Advances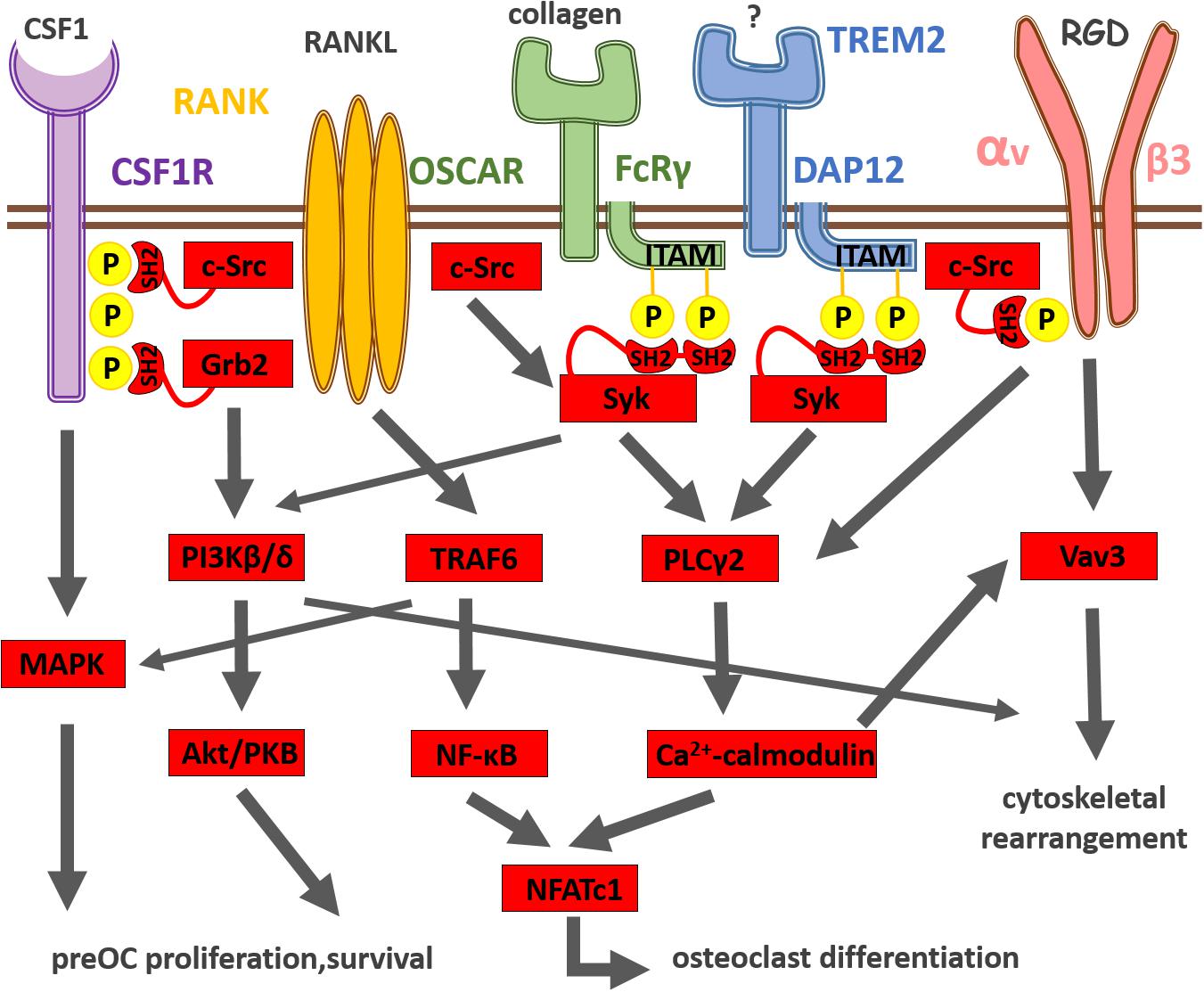
Frontiers | Osteoclast Signal Transduction During Bone Metastasis Formation | Cell and Developmental Biology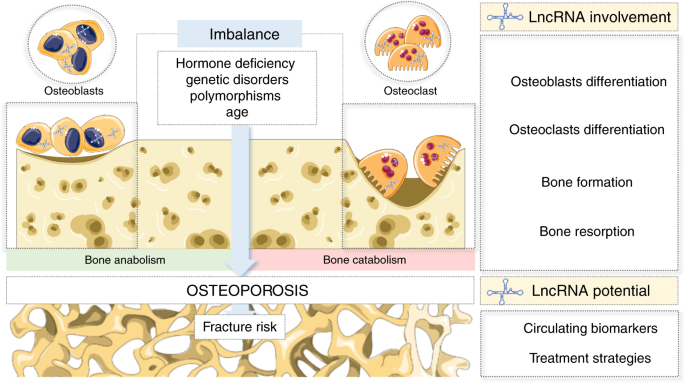
Long noncoding RNAs: a missing link in osteoporosis | Bone Research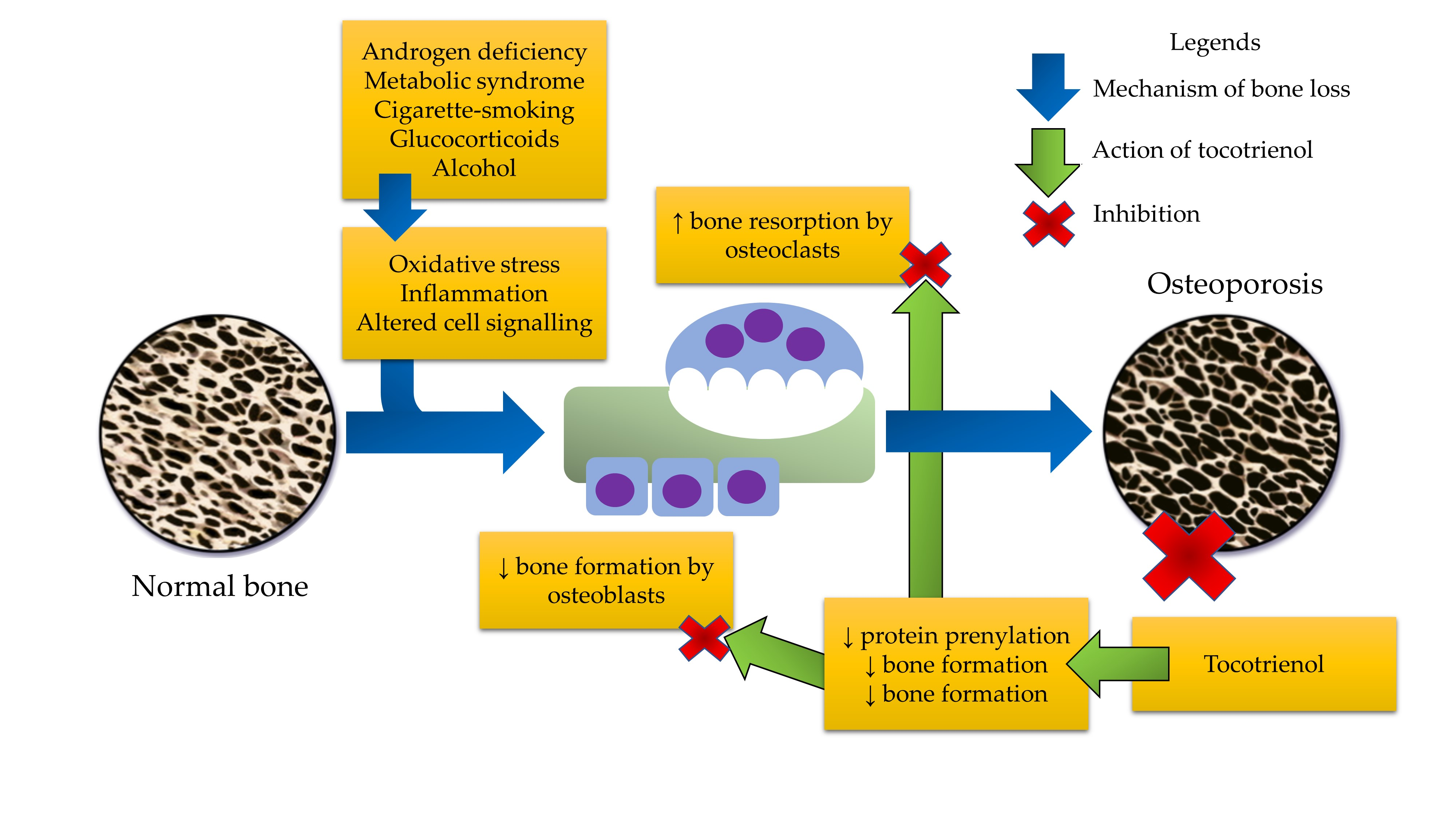
IJMS | Free Full-Text | The Role of Tocotrienol in Preventing Male Osteoporosis—A Review of Current Evidence | HTML
Metformin; an old antidiabetic drug with new potentials in bone disorders - ScienceDirect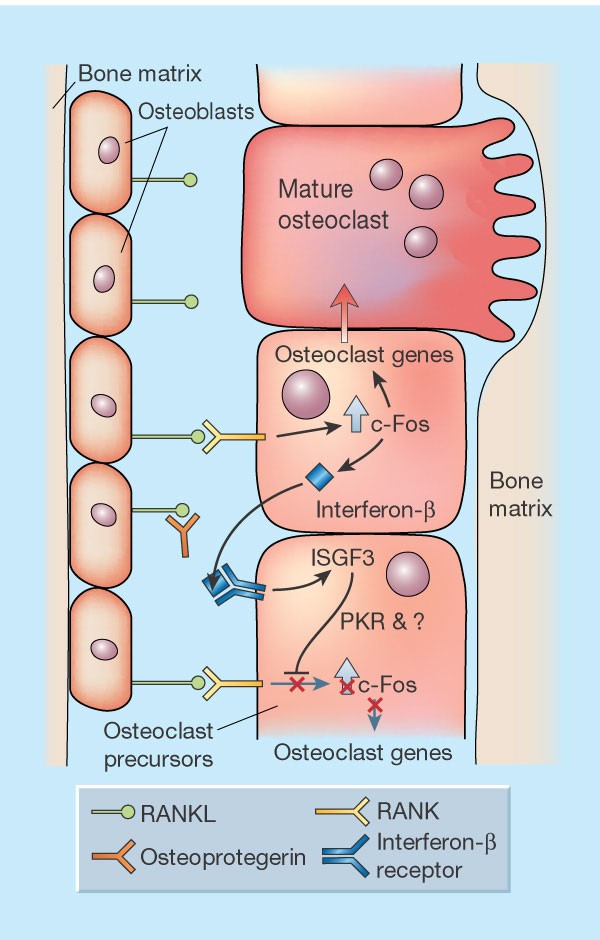
Interfering with bone remodelling | Nature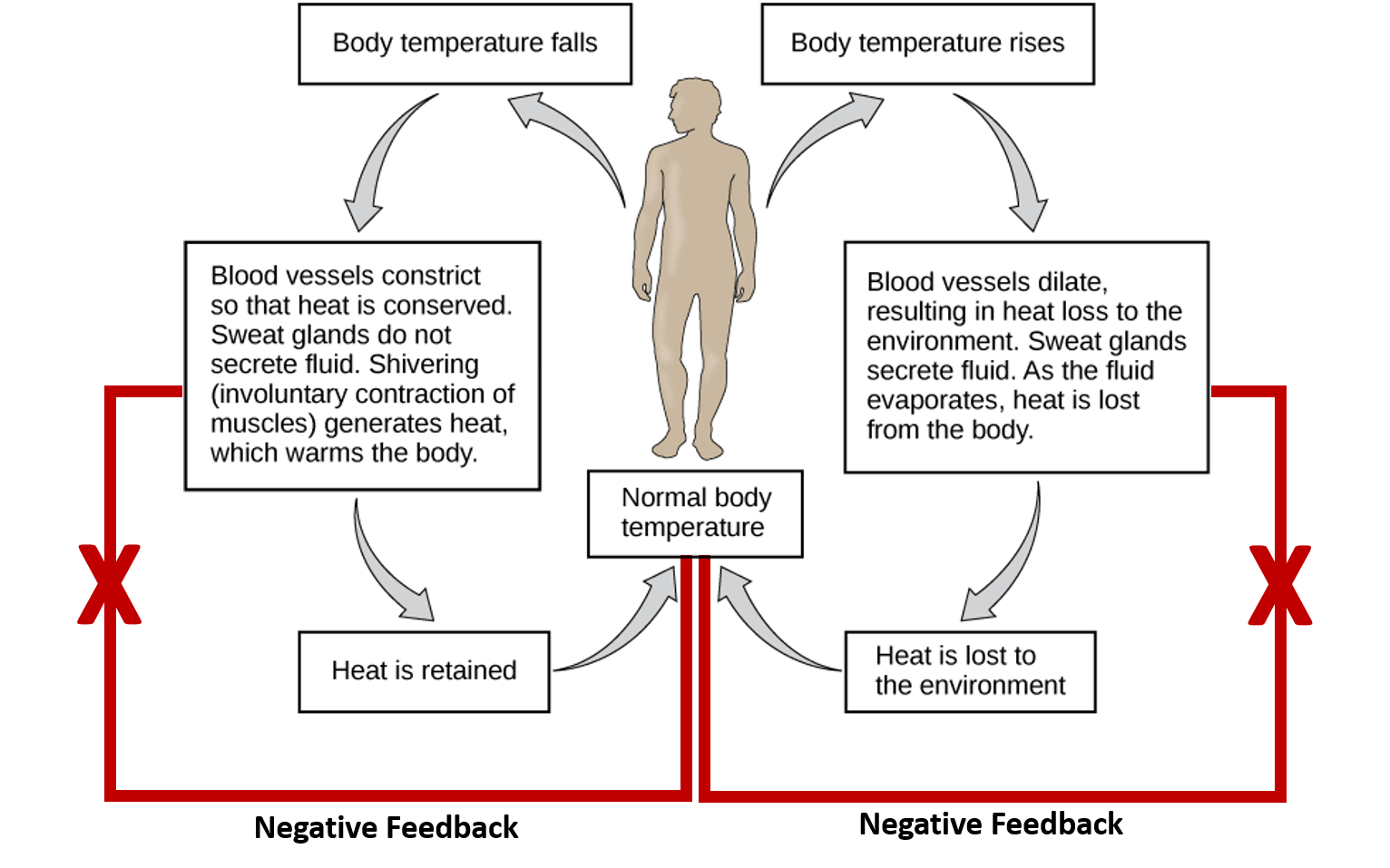
CH103 – Chapter 8: Homeostasis and Cellular Function – Chemistry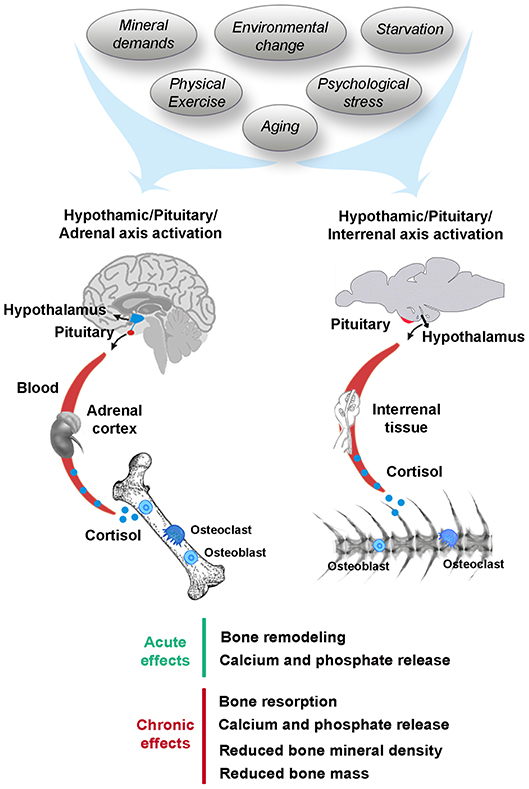
Frontiers | Stress, Glucocorticoids and Bone: A Review From Mammals and Fish | Endocrinology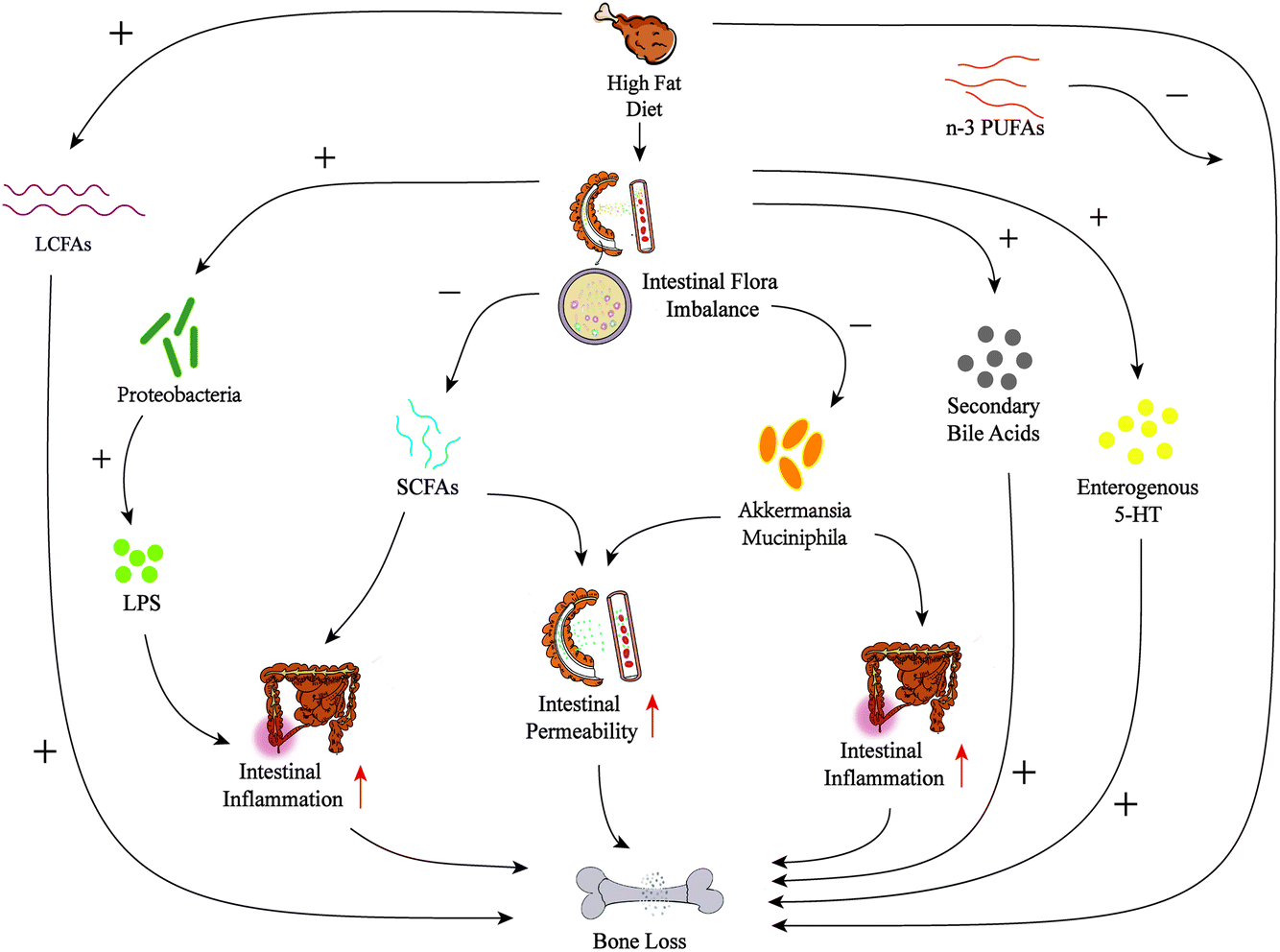
The impact of a high fat diet on bones: potential mechanisms - Food & Function (RSC Publishing) DOI:10.1039/D0FO02664F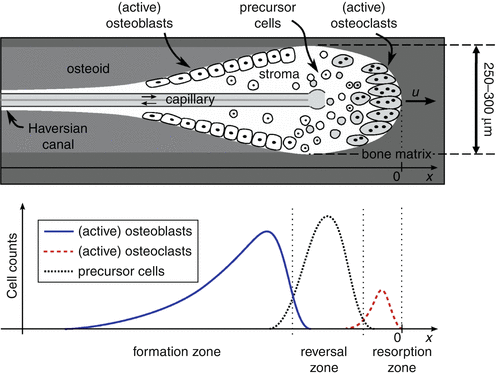
Bone Remodeling | SpringerLink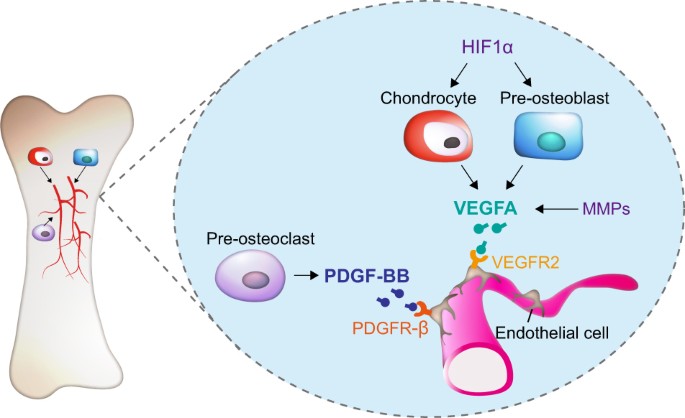
Paracrine and endocrine actions of bone—the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts | Bone Research
Membrane Trafficking in Osteoblasts and Osteoclasts: New Avenues for Understanding and Treating Skeletal Diseases - Zhao - 2012 - Traffic - Wiley Online Library
Bone and bone marrow disruption by endocrine‐active substances - Agas - 2019 - Journal of Cellular Physiology - Wiley Online Library
bones Flashcards | Chegg.com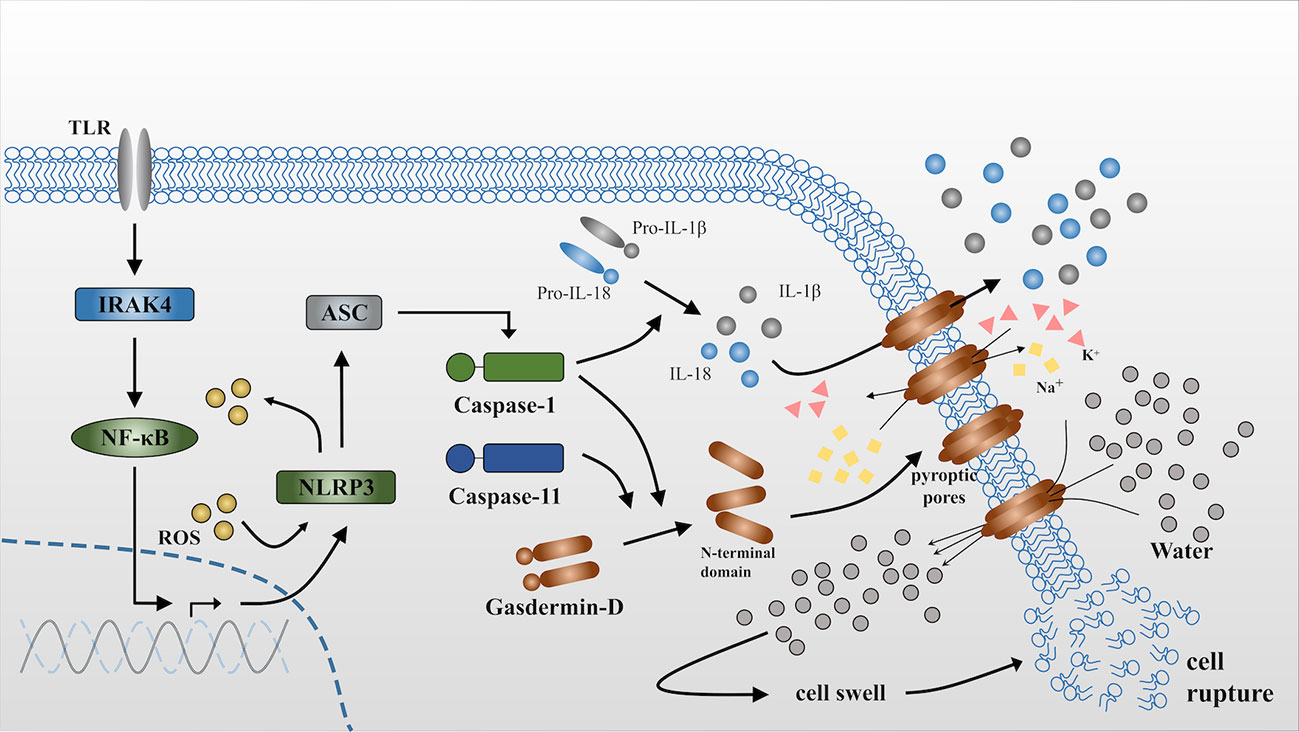
Frontiers | Pyroptosis in Osteoblasts: A Novel Hypothesis Underlying the Pathogenesis of Osteoporosis | Endocrinology
Bone As An Endocrine Organ - Endocrine Practice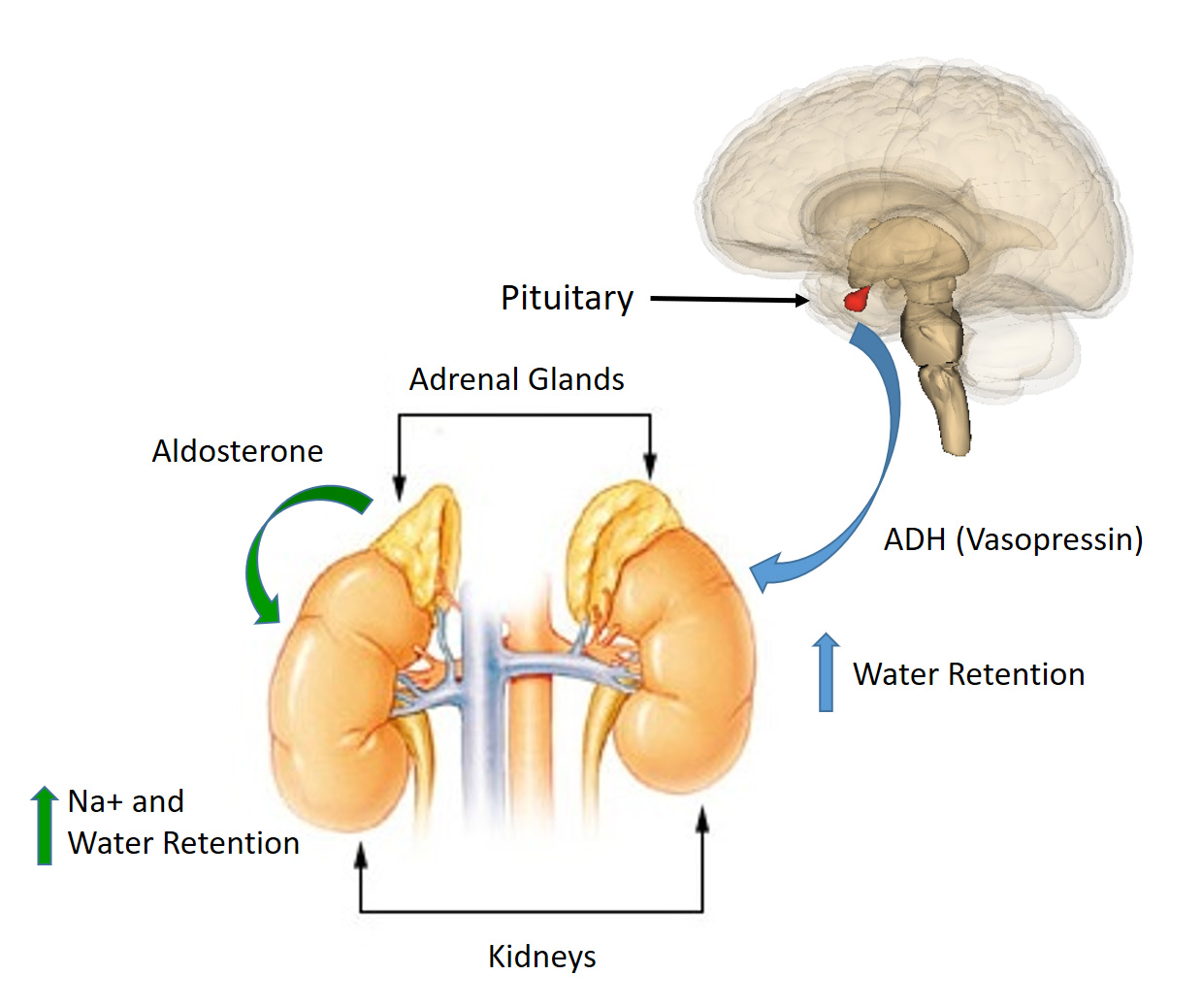
CH103 – Chapter 8: Homeostasis and Cellular Function – Chemistry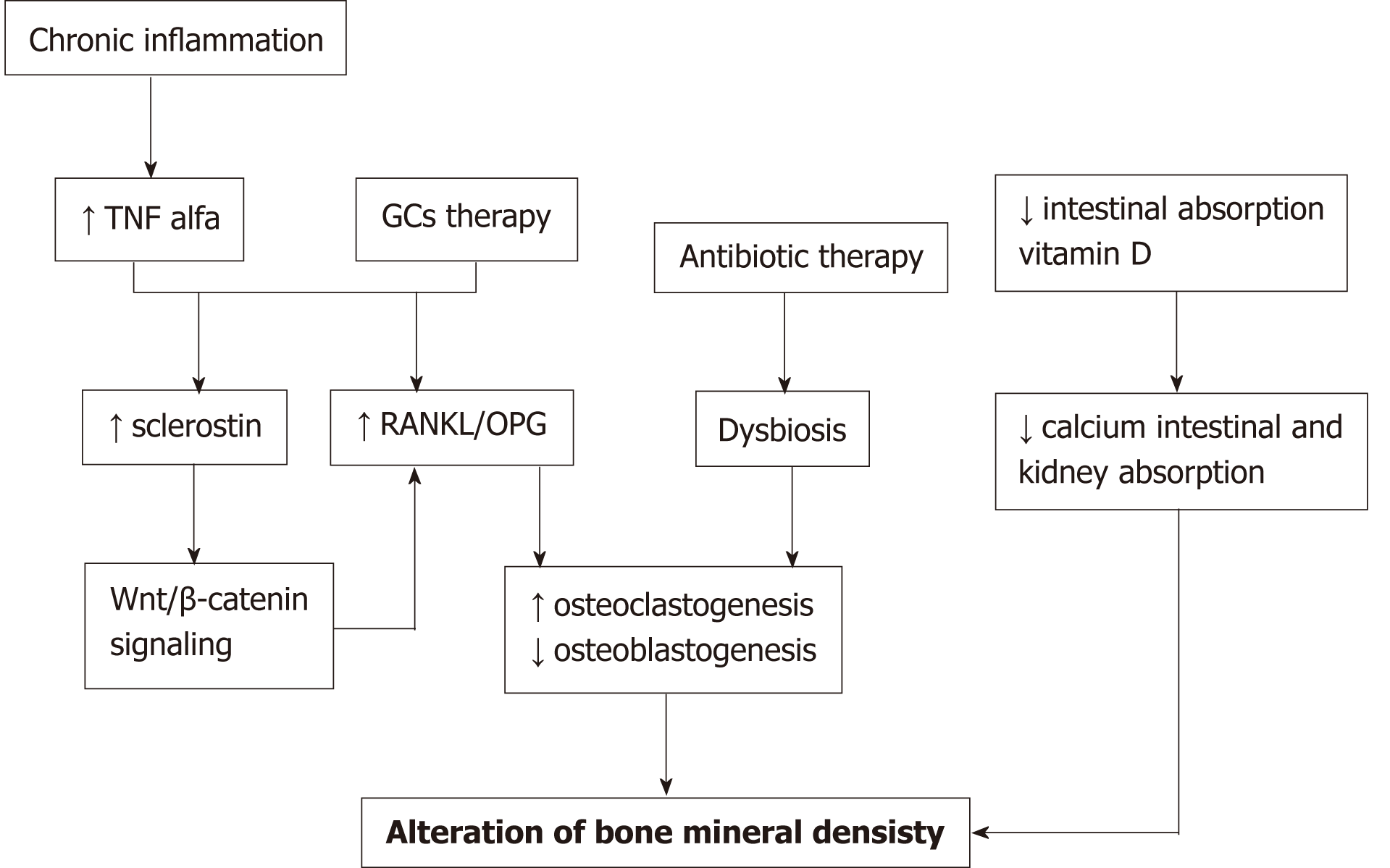
Bone alterations in inflammatory bowel diseases
 Solved: Part A A Homeostatic Imbalance That Activates Thes... | Chegg.com
Solved: Part A A Homeostatic Imbalance That Activates Thes... | Chegg.com






































Posting Komentar untuk "a homeostatic imbalance that activates these bone cells would lead to a loss of bone density."